First things first…
No, aluminum does not rust like other metals containing iron. The reason aluminum is the exception to the rule is the primary ingredient, known as aluminum oxide, chemically bonds to the surface, protecting the metal underneath.
One of the main culprits causing rust is oxygen, and fortunately for the aluminum industry, oxygen is precisely what triggers this firm protective layer to form.
Riddle solved…right? Not so fast.
Does Aluminum Corrode?
Rust is just one form of corrosion.

The colorless, passive oxide film that covers aluminum acts as a barrier against the harsh environments usually responsible for rust in other metals. In seawater, for example, an aluminum alloy resists corrosion 100 times longer than low-carbon steel, according to the 2010 book written by E. Ghali.
Unfortunately, it won’t always result in a happy ending for this helpful metal.
Although rust has no effect on aluminum, there are 10 other types of corrosion that aluminum is vulnerable to. The degree of vulnerability will depend on its alloy content and the environmental conditions around it.
So, let’s get to the heart of it and look at these 10 kinds of corrosion.
1. Corrosion Fatigue
When aluminum and its alloys experience a repetitive cycle of force being applied and removed, the aluminum oxide layer will weaken and become exposed to the eroding environment, causing corrosion fatigue.

For example, imagine an aluminum alloy used on a deep-sea oil rig. This could be found as part of the structure or as a storage container bolted on the outside platform. Regardless of its purpose, the surface metal will be subject to tropical storms and regularly occurring conditions that hammer it with naturally corrosive electrolytes found at sea.
Eventually, the constant barrage will strip away the aluminum oxide layer, allowing the metal underneath to degrade.
2. Crevice Corrosion
Crevice corrosion develops on aluminum when a stationary electrolyte between the passive layer and joining surface decreases oxygen flow and slowly eats away at the aluminum oxide.

This corrosion in aluminum alloys is found primarily in environments consisting of seawater and is less frequently found in atmospheric conditions. Because of the salty environment and the depleted oxygen, corrosion in the crevice is apt to debase the metal ten times faster than it would in an open area.
It takes very little to create conditions ideal for crevice corrosion. This could be metal-to-metal gaps between a nut and bolt or rivets. Or, a metal-to-nonmetal example, like an aluminum alloy resting on a sandy beach, would carry the suitable combination to form crevice corrosion.
3. Exfoliation Corrosion
Exfoliation corrosion in aluminum might also be classified as an example of an extreme case of intergranular corrosion. It’s more common to occur in aluminum alloys that have been specially treated with heat.

When the grain boundaries of the metal are exposed to corrosion, the surface acts in a way that resembles bulging. The top layer of aluminum pulls away from the underlying metal, resulting in exfoliating flakes.
4. Filiform Corrosion
Filiform corrosion in aluminum is easily recognizable by the thin channeling under the surface, which primarily damages the appearance of the metal rather than the actual integrity.

More often than not, filiform corrosion takes place when the aluminum has no protective layer, or the applied coating is flawed, allowing moisture to ruin its surface.
5. Fretting Corrosion
Be careful! This form of corrosion is easily confused with fatigue, but it’s not the same at all.

Where corrosion fatigue depends on the heavy pressure being applied and removed in frequency, fretting results from uninterrupted contact and slight vibrations between aluminum and the heavy load.
As an example, this constant contact and motion could take place in trusses where bolts under heavy stress secure two parts of a structure prone to some movement.
6. Galvanic Corrosion

Galvanic corrosion forms when two metals in contact with differing chemical makeup are exposed to a conducting element.
The two varying metals are usually perfectly fine in contact with each other depending on their surroundings. But if you take a structure where a joint combines aluminum and stainless steel and place it in the ocean, seawater is the perfect catalyst to cause a galvanic reaction.
7. Intergranular Corrosion
According to Dr. Dmitri Kopeliovich, intergranular corrosion occurs in aluminum alloys containing zinc, copper, iron, and magnesium.
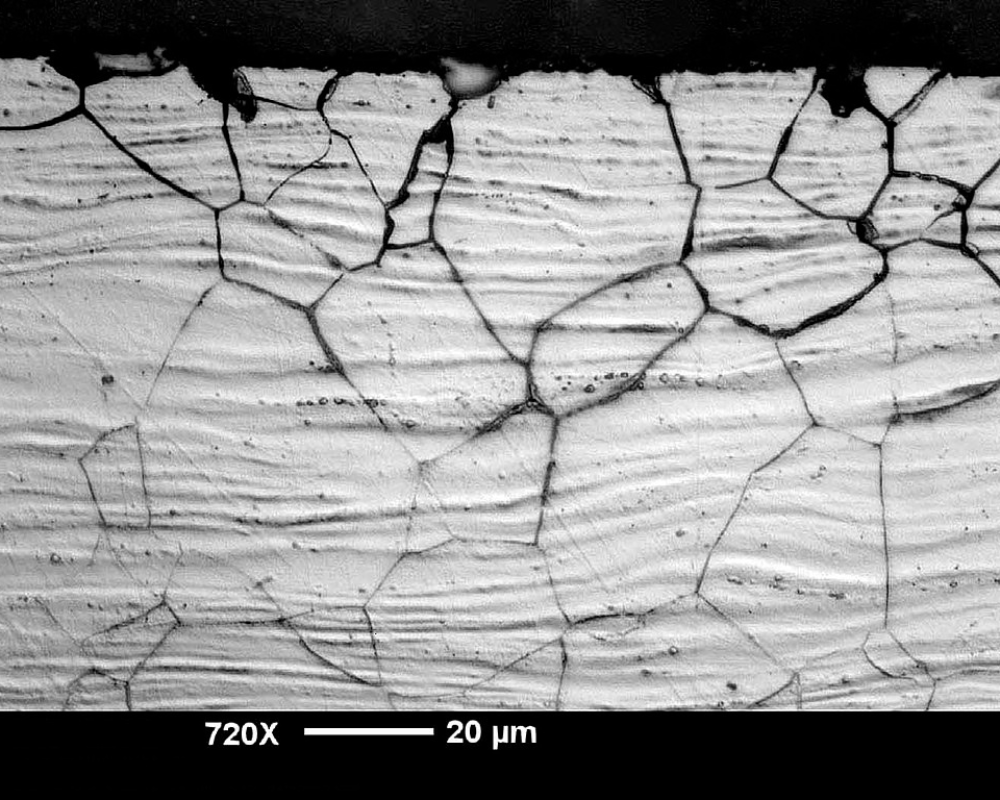
Like its more severe occurrence, exfoliation corrosion, intergranular corrosion attacks the grain boundaries of fortified aluminum alloys. But instead of pulling the top layer apart from the body, the crystalline structure will show signs of cracking.
8. Pitting
Pitting is a common form of corrosion that metals with thin passive layers experience when exposed to watery environments. In aluminum, pitting appears as little cavities of all sizes in a localized area of the surface metal.

If you notice pitting, don’t discount the presence of other types of corrosion in the same area. Despite the smaller size of these cavities, pitting is considered one of the most harmful types of decay because its not so easy to recognize. Considering these holes will often fill with oxide, the weight loss may be too catastrophic by the time you realize they’re there.
9. Stress Corrosion Cracking
Stress Corrosion Cracking is a subcategory of intergranular corrosion and is tough to get a hold on. Several factors contribute to SCC, and when this type of failure occurs in aluminum alloy, it happens without warning.

The destructive growth of cracking caused by SCC is made possible by two variables working together simultaneously: environment and tensile stress.
It’s important to note that the tensile stress applied to induce stress corrosion cracking is far less than its manufactured breaking point.
10. Uniform Attack
A uniform attack is considered the most general form of corrosion and spreads evenly over the entire surface of the metal when exposed to an imbalance of pH levels.

Because oxidation rates of this standard corrosive will progress over measurable time until metal failure, the lifespan of uniform attack corrosion can usually be accurately predicted.
Better yet, it can also be prevented!
What Causes Aluminum to Corrode?
Several factors lead aluminum to corrode, but in its most basic understanding, what causes aluminum to corrode is moisture, the alloying metals, and the contributing variables found in its immediate environment.
I’m not trying to be purposefully vague, but your answer requires more explanation. And I don’t know if the science behind it is what you’re looking for. However, after some time researching Ghali’s book, I’ve got the finer points for you to consider about what causes aluminum to corrode. It’s not a complete list, but you’ll learn all about the big-time influencers.
Water

- pH levels outside the range of 4.0 through 9.0, along with other contributors like water temperature, will drastically change the corrosion resistance of aluminum by affecting the passive layer.
- Wet soil with imbalanced pH levels (containing acids and alkaline) will increase the rate of attack on metal.
- Seawater often leads to pitting. But the deeper the metal goes, the worse the crevice corrosion gets. However, the 5xxx series of wrought aluminum alloys tend to last longer than others.
Metal
- Depending on how the metal was treated can affect the type of corrosion that is capable of occurring.
- Certain alloys have better resistance to particular corrosion.
- Nickel can enhance pitting.
- Antimony adds higher resistance to salt water.
- Cadmium and chromium can increase the resistivity of some aluminum alloys.
- Lithium and copper can decrease the resistivity of some aluminum alloys.
Environment

- Water where aluminum experiences high flow rates lead to more chance of corrosion.
- Unlike well water and seawater, sterile solutions will positively affect the corrosion rate.
- Circulating water systems where chromates and phosphates are present inhibit any corrosive reaction.
- Chlorides, sulfides, and heavy metals cause pitting and other localized forms of corrosion.
- Corrosive staining is possible when water vapor is present in the atmosphere.
- The lack of oxygen dramatically limits the growth of corrosion.
How Long Does It Take for Aluminum to Corrode?
Sorry to say, but it depends.
Aluminum could start to corrode in a few hours or a few years. It’s entirely dependent on the condition of the metal and its surroundings.
If the passive layer is intact and the metal sample is in reasonably good (non-corrosive) states, the aluminum’s integrity could still be strong in another 40 years. But, in that same scenario, except that the aluminum oxide has been scratched, degradation could take over in a matter of months. Even faster if moved to another location.

Another factor that could extend its fight against corrosion is whether or not you take steps to protect it.
How to Protect Aluminum From Oxidation?
The most popular method to protect aluminum from oxidation is to anodize it. There are other ways, but anodizing is as effective as they come.
Anodizing protects aluminum from oxidation by an electrochemical method of submerging aluminum in an electrolyte bath. By passing a current through the solution, the metal forms a strong aluminum oxide coat that’s resistant to corrosion, peeling, and flaking.
According to the UK’s Aluminum Federation, which represents their aluminum industry and businesses, four commercial use anodizing options exist.
- Sulphuric Acid Anodizing
- Integral Color Anodizing
- Chromic Acid Anodizing
- Hard Anodizing
How Long Does Anodized Aluminum Last?

If your aluminum is anodized, it can help it last upwards of 100 years with the proper care and coat thickness.
You see, the thicker the coat, the longer the anodized aluminum’s life. Eventually, the coating will thin and lose effectiveness due to the elements around it. But replacing the anodized coat is easy and provides a new lease on life for your aluminum.
A report written by an anodized aluminum label named Qualanod gives us actual data behind the expected timeline we might project. After comparing samples with varying thicknesses and in varying environments, Qualanod summarizes the findings as follows:
- The samples with the thinnest protective coat showed minimal loss over 17 years.
- The samples with the highest rate of thickness coating nearly doubled in thickness loss compared to the previous example in only 5 years. However, the location was known for high pollutants from steel manufacturing.
- Loss of thickness decreased over time.
Now 17 years is far from the 100 I stated. It may come down to safety guidelines or simply the change in environment, but a longer lifespan is possible. Our economy has been using anodized aluminum since the 1930s, and some buildings are still around with no signs of corrosion.





