Seeing as you and I recently demystified corrosion and the 14 metals impervious to rust, why not look at the metals that do rust? It’s a very short list.
While you’ll find many metals of different names, colors, and physical attributes with signs of corrosion, only iron is capable of rusting.
Remember, rust is just one type of corrosion that diminishes the iron content of any metal through an electrochemical process. The spark needed to ignite that process is exposure to oxygen and water.
Now, this can be confusing. You might have some old antique sitting in your garage that looks nothing like iron and is covered with rust. Are you sure it’s rust? It certainly could be. But let me show you how.
What Are the Two Main Categories of Ferrous Metals?

In case you need a refresher, there are two types of metals, ferrous and non-ferrous.
We’ll focus on ferrous metals since these are the only metals capable of rusting. More importantly, they can rust because ferrous metals, as their name implies, contain iron. Non-ferrous metals, simply enough, are metals with no iron content.
However, when discussing ferrous metals, there are two main categories accepted in and outside the world of science: iron and steel.
I know, I know. I assured you that iron was the only metal that rusts, which is technically accurate. But steel is an abundant metal used everywhere in our world. So much so that steel and its alloys are recognized as an entirely separate category of ferrous metal.
And because steel is an iron alloy with no more than 2% carbon, it has the properties necessary to induce the chemical reaction that causes rust.

There are other names in this family of ferrous metals. A touch more carbon and steel becomes cast iron. Start adding different mixtures like nickel, chromium, zinc, or tungsten, and you start recognizing other metals that might match the antique in your garage.
Rusting of Iron
Before we get down a rabbit hole of alloyed metals that may eventually rust, let’s focus on the two main categories they derive from. First up, iron.
The rusting of iron is the effect it has when coming into contact with water and air. Rust is corrosion specific to iron and eats away at the integrity and toughness of any metal with any trace of it.
Other forms of corrosion merely form as an extra layer without weakening the material. Some alloys with lower iron content have other transitional properties that resist the effects of rust for extended periods. But rust can eventually take hold, like in stainless steel, once its chromium protection has worn down.
Does 100% Pure Iron Rust?

I’ve seen references online suggesting that pure iron doesn’t rust. Unfortunately, that isn’t true.
100% pure iron will rust quickly once oxygen and moisture are introduced to this smooth and pliable element. Even faster than iron with slightly less purity.
Pure iron isn’t what you think it is. It’s not the iron ore used to manufacture steel and other durable materials. To really make use of its potential, you have to mix it with other alloys, allowing it to bond with other beneficial attributes.
The misunderstanding about pure iron not rusting comes from iron that has been well-kept. Rusting won’t occur on its own. Iron doesn’t have a shelf life. It’s the exposure to the elements without proper care that eventually gives in to the electrochemical change.
I’d be amazed if you got your hands on 100% pure iron. It’s incredibly rare to discover it in its purest form. And the pieces found have come from igneous rocks near past volcanic eruptions, less easily discoverable than your backyard.
Does Steel Rust?

Steel is the second main category of ferrous metals, and even though its iron content is slightly lower, it can and will rust when exposed to air and water.
In today’s world, 80% of the steel produced is carbon steel, which is an iron alloy with roughly 1% of carbon. The good thing about steel is that it’s naturally resistant to corrosion because of its impermeable texture. And with the small addition of carbon and other protective coatings, it’s more difficult to physically damage this metal, which would leave it more vulnerable to oxidation.
However, steel isn’t invincible. It will rust with enough time and external pressures because of its iron origin.
Does Alloy Steel Rust?
Alloy steel, like carbon steel and any other iron alloy, will rust, given enough time and interaction with destructive environments. The only difference with alloy steel is that the variation of other blended metals will determine how resistant it is to corrosion, specifically rust.
So even if the steel alloy is safer against rusting degradation by available barriers like chromium or molybdenum, harsher conditions like salt water in the oceans will eventually break through.

Although, if the alloy steel is cared for and kept clean and safely conditioned, it’s possible to extend its life expectancy and delay rusting indefinitely.
How Long Does It Take for Steel to Rust?
The length of time it takes for steel to rust isn’t a one fits all solution and is entirely dependent on the circumstances of the environment.
There are various methods to test the corrosion rate of steel, and iron for that matter, including the popular estimation of metal weight loss before and after exposure to a setting. By dividing the result by the calculated observed area, and the time it inhabited the conditions, you can narrow down the corrosion rate.
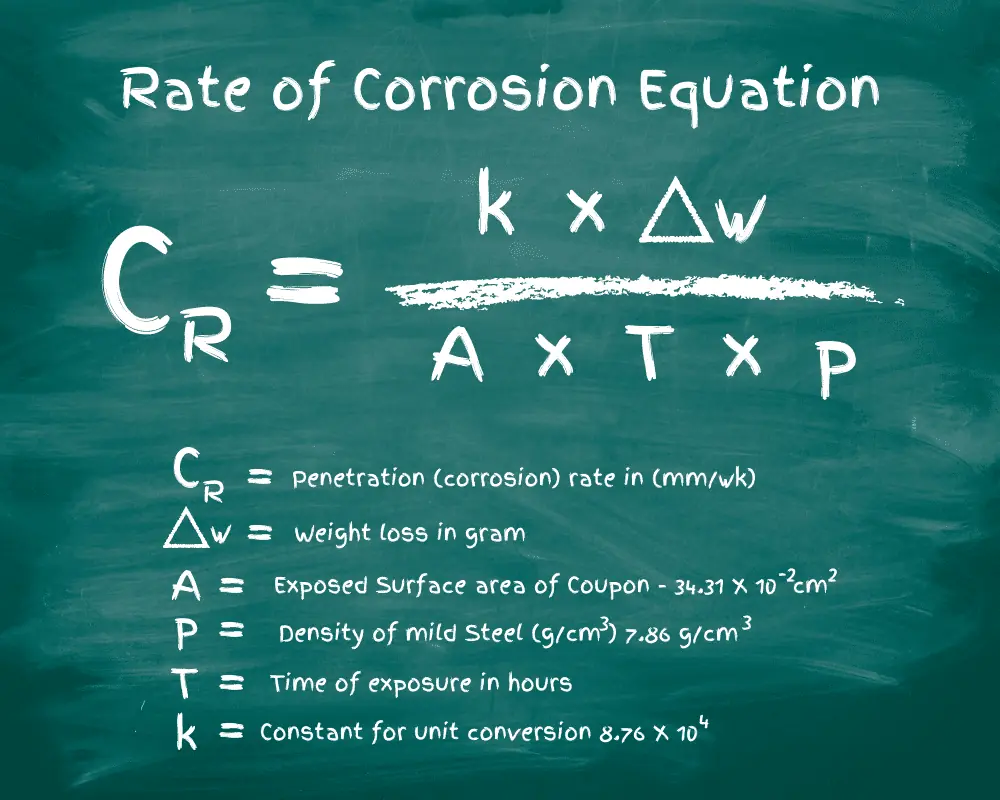
The problem with this is there are too many contributing factors to settle on a uniform rate. No test area is the same as another. PH levels change, and moisture collects differently with different elevations.
A 2016 article by the Journal of Scientific and Engineering Research (JSAER) details the study of corrosion rates on mild steel in five different environments: hydrochloric acid, underground (soil), atmosphere, salt water, and fresh water.
Each week of the six-week trial, scientists analyzed the mild steel for all forms of corrosion. Here are a few of the observations made by the research team.
- The rate of corrosion casts a broad scope between the five testing backdrops.
- The salt water and soil tests showed a rapid increase in corrosion after the third week. Unfortunately, the salt water results reliability is difficult to say since the researchers never recorded the salt levels.
- Mild steel in the atmosphere showed the minimum trace of rusting. But researchers noted that the particles in this particular space were relatively clean of emissions found elsewhere.
- The fresh water sample showed an initial bump in corrosiveness yet settled to a steady growth due to the low volume of ions present.
- The hydrochloric acid was the most impactful environment on the rusting of mild steel. It showed an initial drop but a noticeable spike in corrosion rate after week 2.
Some intriguing conclusions come from the results, but applying these as a general rule would only work in identical conditions. You can see that some of these trends had external factors that significantly changed how fast the steel developed rust.





